 INTELLIROD SPINE: $1 MILLION FOR WIRELESS SENSORS (Orthopedics This Week)
INTELLIROD SPINE: $1 MILLION FOR WIRELESS SENSORS (Orthopedics This Week)
Also Read my interview with the CEO… 6 Questions with Ric Navarro, a serial technology innovator in the spine space
Curious about the strain being put on that spine implant? So was Intellirod Spine. The company has just announced that it has raised more than $1 million in equity financing from new and existing investors to do that and more.
As indicated in the July 26, 2016 news release, “Funds will be used to reach key milestones toward the commercialization of the company’s sensor technologies and related lumbar fusion implants.”
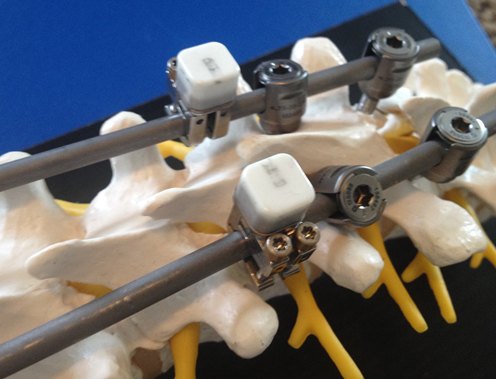
According to Intellirod CEO Ric Navarro, “The LOADPRO gives a surgeon new and important information for improving surgical technique. The device becomes an adjunct to the surgeon’s tactile feel for avoiding excessive implant loading.”
Rolando M. Puno, M.D. of Louisville, Kentucky, stated, “With LOADPRO, for the first time, spine surgeons will have an accurate tool to measure and determine the amount of force being applied to the rod implant as spinal deformity is being corrected. Knowing the amount of applied strain will allow spine surgeons to adjust their surgical techniques to achieve the best possible correction of deformity. Furthermore, spine surgeons will be able to avoid the risk of inadvertently applying excessive strain causing fixation failure and/or loss of correction.”
Spine surgeon Michael Steinmetz, M.D. further explained, “The information gained from LOADPRO may allow the surgeon, for the first time, to understand the forces applied to spinal constructs when performing deformity surgery. This information can lead to improved surgical results and patient outcomes.”
Says the news release, “In addition, the company has completed its pre-clinical testing in support of an FDA application for ACCUVISTA, its implantable version of the product. The ACCUVISTA sensor monitors post-operative strain in rods in degenerative disc disease fusion patients, and the company is bullish on its new application for ACCUVISTA in children with scoliosis.”
Asked for detail on the “key milestones” they are aiming for, Ric Navarro told OTW, “We are moving toward FDA approval of LOADPRO, the first clinical use of LOADPRO and CE Mark for LOADPRO.
“Surgeons currently assess forces by feel, this will allow them to quantify forces (by way of rod strain) as they do their correction in kyphotic deformities including lumbar flat back. Keeping forces lower while still achieving the desired correction is expected to lead to fewer hardware related complications.
“We are looking to finish out the fundraising round (through September) to enable pursuit of a Humanitarian Device Exemption and a pilot IDE [investigational device exemption] trial in children with scoliosis. Since LOADPRO is very similar in construction to ACCUVISTA we feel it will pave the regulatory pathway through FDA as a Class II device.”