How medical-device CEOs can navigate digital disruption in healthcare (McKinsey & Company)
Medical-device companies will need to reinvent themselves to stay competitive. Now’s the time to craft a strategy and scale a transformation.
Digital has already disrupted major sectors of the economy, and a revolution is underway in healthcare. As in other industries, battlegrounds are emerging, and there will be clear winners and losers. Medical-device players, facing unique opportunities and headwinds, will need to reinvent themselves as digital health companies to remain relevant and win in this fast-evolving market. Recognizing the urgency, almost all of the industry’s CEOs have declared digital health a top priority.
An earlier article, “Four keys to successful digital transformations in healthcare,” discussed broad trends and emerging battlegrounds—building direct relationships with consumers, finding new sources of value, collaborating for complementary capabilities, and contributing to burgeoning industry standards. Building on that work, this piece outlines how medical-device companies can define a winning strategy and design an at-scale digital transformation.
Where to start: Understanding stakeholder actions
Medical-device CEOs and boards recognize the power of digital and advanced analytics, and they’re investing in related capabilities. But for big changes to take hold and deliver lasting impact, they need to be woven into the organization’s DNA, including its culture and processes (for more questions to think about before a transformation, see sidebar “Preparing to win”).
Winning digital health companies will change in several ways at once: they’ll provide consistently delightful experiences to all customers, including patients, caregivers, clinicians, and providers. They’ll create a wide array of intelligent, personalized products that deliver demonstrable clinical value, and they’ll use insights on demand to take on appropriate risk with providers, payors, and regulators. They’ll also reimagine processes to dramatically reduce costs and accelerate decision making.
How do they get there? Rather than turn inward, we believe industry leaders should constantly evaluate the actions of stakeholders across the value chain to define the pace and direction of their responses in this frothy market, as shown in Exhibit 1.
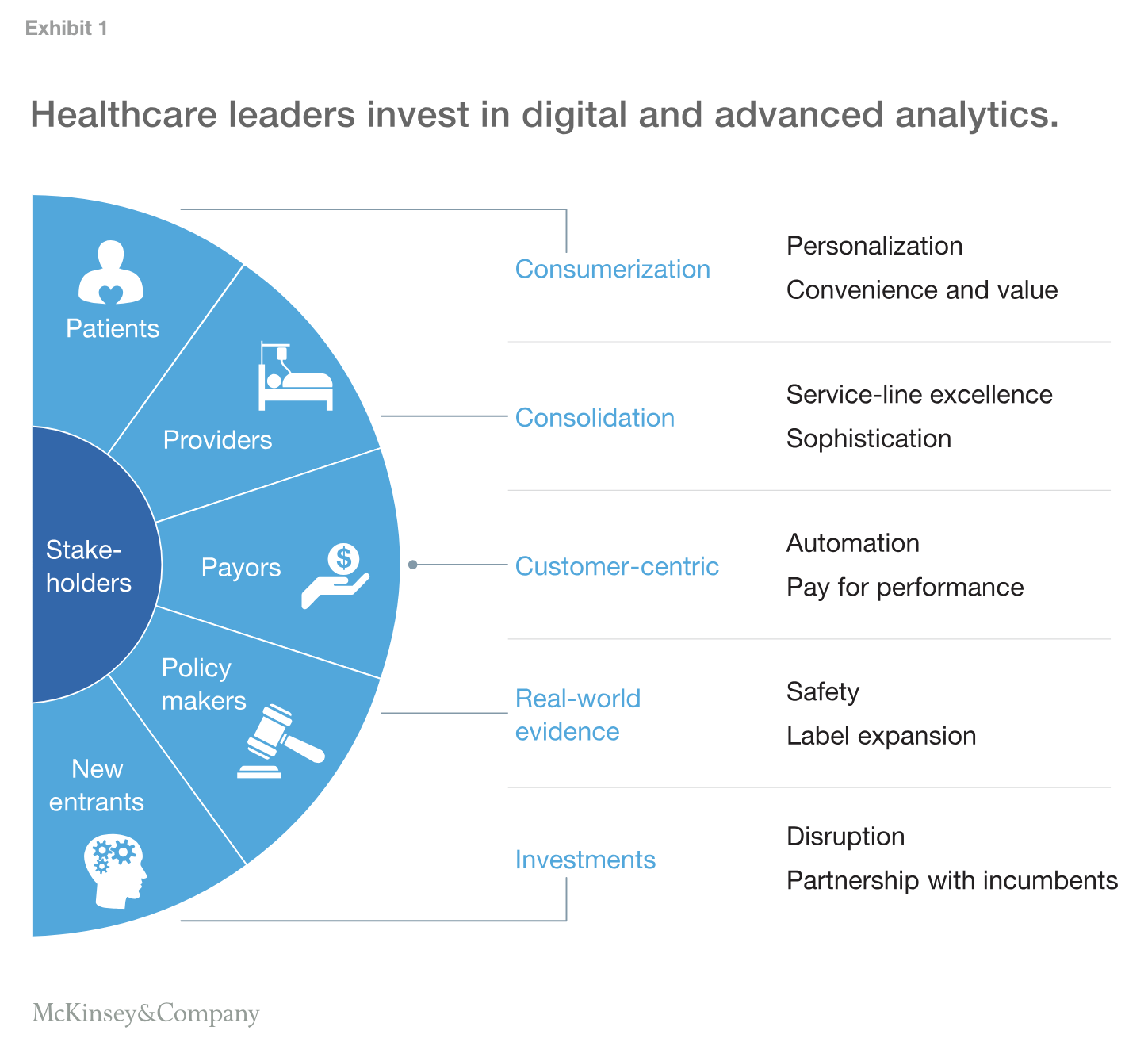
Providers are scaling rapidly and making care delivery more sophisticated. Roughly 150 systems in the United States have established significant leverage and reach. They are vertically integrating to create clinical service lines that drive outcomes, manage risk, and create profit. Scale also allows providers to drive evidence-based medicine and create novel risk-sharing arrangements with manufacturers to control costs and improve outcomes.
Providers are also making the transition from offering B2B services to clinicians to B2C services for patients. As more clinicians are employed by hospitals, their ability to exercise their preferences is decreasing. In parallel, providers are moving beyond housing in-patient care to higher sources of value such as alternate-care settings and services, and monetizing assets, including data and patient access. All these changes have significant implications for medical-device companies’ innovation, offerings, services, and commercial models.
Payors are innovating while managing the bottom line. Those that have historically been viewed as B2B financial-underwriting entities are rapidly reinventing themselves as B2C organizations where members are a major source of revenue and digital is a critical capability.
For example, in the United States, led by the Centers for Medicare & Medicaid Services (CMS), payors are reducing costs through value-based programs. By 2020, CMS expects about 75 percent of contracts to be pay-for-performance. These changes are creating opportunities for innovative partnerships, raising the bar to demonstrate the value of products and services in real-world settings.
Policy makers are harnessing big data to assess safety and efficacy. In the United States, the Food and Drug Administration (FDA) is establishing a National Evaluation System for Health Technology (NEST) to “quickly identify problematic devices, accurately and transparently characterize and disseminate information about device performance in clinical practice, and efficiently generate data to support premarket clearance or approval of new devices and new uses of currently marketed devices. Essentially, NEST should be of, by, and for the medical device ecosystem and configured to provide maximal value to stakeholders, including the critical data needed by the FDA to make decisions that currently must be made with less comprehensive information.”1
This system brings together data from diverse sources and initiatives (for instance, PCORnet, Sentinel, registries, health plans, and delivery systems) to create a central mechanism to answer priority questions.
Each R&D organization will need to build new capabilities to succeed in this changing regulatory environment.
New entrants are flocking to healthcare. Large technology players and venture-capital-backed start-ups are bringing disruptive Silicon Valley mind-sets, speed, architecture, and investments at scale. Rather than posing an outright threat, tech companies offer healthcare incumbents many opportunities, as these companies are aware of their gaps in understanding of clinical and regulatory practices and are open to partnering with established healthcare companies (see sidebar “Examples of innovation”).
Imperatives for medical-device players
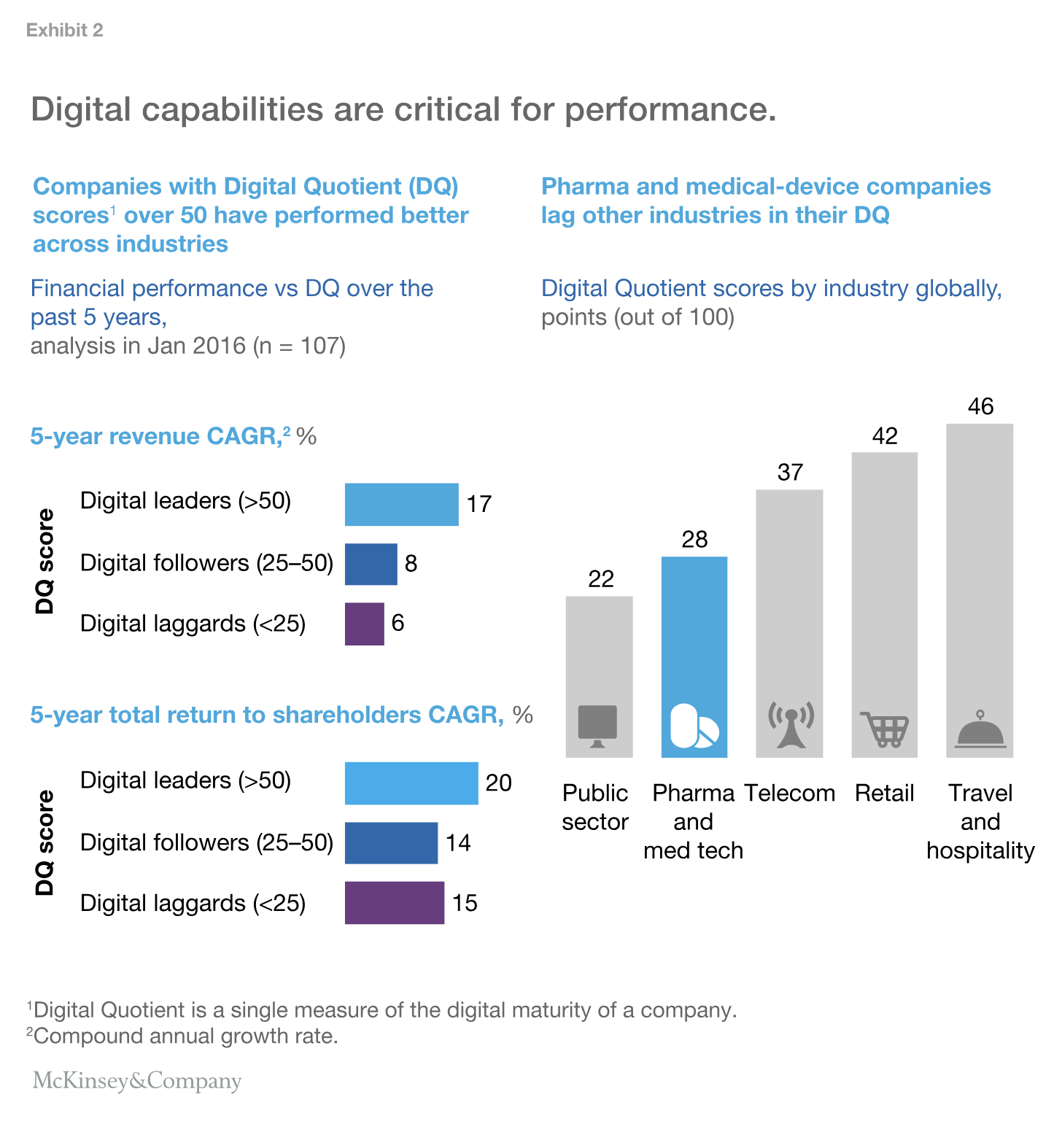
These changes across the value chain are collectively creating a “burning platform” that is compelling medical-device players to scale up their digital and advanced-analytics capabilities. Our cross-industry benchmarking tool, Digital Quotient, shown in Exhibit 2, demonstrates that digital capabilities are critical for performance, but medical-device and pharmaceutical companies’ capabilities are far behind those in other sectors.
To understand how to improve this dramatically, we analyzed the full spectrum of opportunities across the profit-and-loss statement of a typical medical-device company and identified more than 200 use cases. These can be organized in four broad themes.
Get closer to customers
As patients become more central to success, leading companies will need to understand, engage, and influence individuals better, just as the leaders do in consumer tech, retail, and consumer packaged goods.
Hospitals, who are customers for medical-device manufacturers, are also evolving. Winners will need to fine-tune their commercial engines to optimize their cost-to-serve and go-to-market models.
Example use cases:
- Consumer engagement. Create a 360-degree view of patients connecting healthcare, environmental, and behavioral information to better understand what drives outcomes and to deliver timely, personalized microinterventions. We’ve seen players grow revenue by up to 10 percent using digital ecosystems that engage patients deeply.
- Commercial-spend optimization. Optimize microtargeting to reach the right physicians and providers based on volumes, outcomes, risk profiles, purchasing behavior, and disease-incidence rates. In addition to lowering cost to serve by 15 to 20 percent, this approach identifies new customers to drive growth.
Build intelligent products and optimize the innovation engine
Winners will rethink all aspects of their models—offerings, go-to-market approach, and R&D operations—to capture new sources of value, drive efficiency, and compete in the changing market.
Example use cases:
- Intelligent products and solutions. Wrap digital solutions around products to deliver better outcomes. These can span predictive diagnostics and early detection in imaging, fully digital operating rooms, and remote patient care in therapeutic areas such as cardiovascular, diabetes, and mental health.
- Digital clinical trials. Optimize site performance by accelerating trial timelines and reducing costs, including better site selection, country footprints, and protocol optimization.
Assume more risk as appropriate
Winners will define novel risk-sharing relationships with providers. Some manufacturers have already begun to embrace new models of contracting (for example, early evidence in the bundled hip-and-knee markets), risk sharing (such as St. Jude Medical’s rebates for cardiac resynchronization therapies in case of required revision), and solutions (for example, Stryker’s subscription-based analytics packages) for providers. These require robust approaches to measure, track and underwrite outcomes in the real world.
Example use cases:
- Value-based care. Collect and analyze data to track the total cost of care, patient outcomes, and patient satisfaction to develop creative manufacturer/provider contracting models. For example, a typical implant represents 15 percent of the total cost of the joint-replacement episode. Manufacturers are working with providers to optimize the remaining 85 percent (for example, inventory, operating-room utilization, and supplies).
- Real-world evidence (RWE). Create a platform to collect, integrate, analyze, and report real-world data to prove treatment efficacy, safety, and value. Taking ownership of RWE can help create new markets, such as for 3-D mammography.
Reimagine processes
Reimagine and automate end-to-end processes to drive efficiency and unlock new insights. Federal healthcare agencies recognize this opportunity as well and have made important strides in their journey.
Example use cases:
- Digital supply chain. Optimize supply-chain costs and improve customer service using data-driven insights into inventory levels and sales forecasts. A smart digital-inventory-management process helped a leading device player raise inventory efficiency by 15 percent.
- General and administrative (G&A) automation. Digitize core G&A processes to create greater levels of efficiency and effectiveness. Leaders have captured 20 to 50 percent improvements in cost and efficiency.
Each of these changes has the potential to create significant value, and together, we estimate these advances can help leaders improve earnings by 15 to 30 percent. However, getting the full value of the opportunity will require embedding digital and advanced analytics in all aspects of operations, at scale.
How to design and execute a digital transformation at scale
The companies that succeed will move away from a bottom-up “let a thousand flowers bloom” approach and design a top-down at-scale transformation. The four keys for the transformation are shown in Exhibit 3.
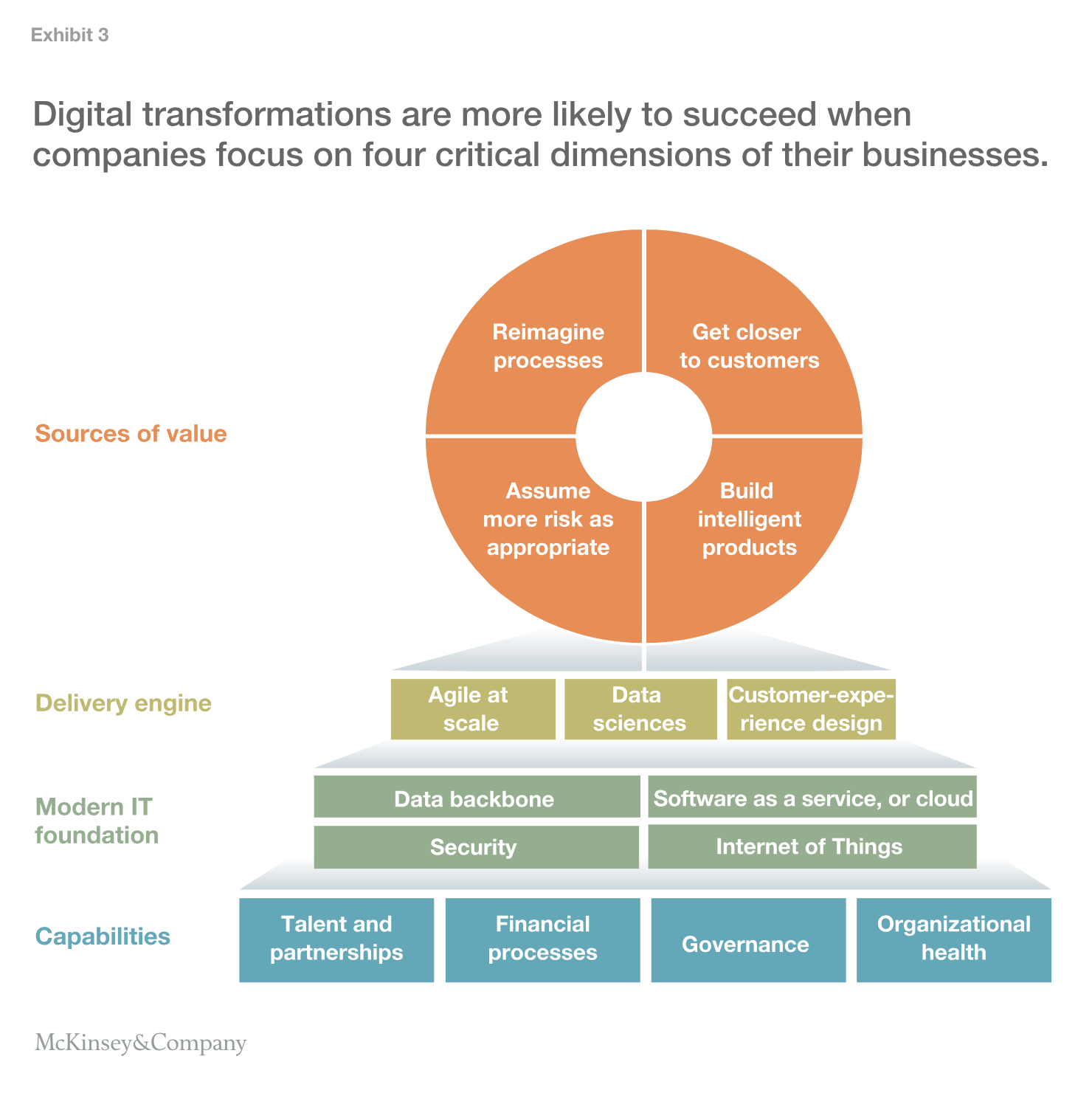
All large healthcare companies are trying to transform themselves around these four keys. One such company is Johnson & Johnson, and its journey is described in an interview with its chief information officer, Stuart McGuigan. He talks about how the company decided to double down on the use of technology and build a flexible but secure digital IT organization supported by the cloud to enhance the development of smarter healthcare products and to improve customer and patient experiences with the company.
Several factors enable at-scale transformations such as the effort at Johnson & Johnson. These include clearly recognizing digital and analytics as a strategic priority and aligning the top team on themes for focus—for instance, intelligent products, customer engagement, and analytics on demand. It’s also important to invest in modernizing the IT foundation (for example, moving 85 percent workloads to the cloud or creating an enterprise-wide data foundation) and to stand up core enablers to manage the transformation (for example, reorganizing and hiring external talent, and developing an ability to launch and successfully manage every project as agile). Another key element is using the foundation to build value-creating innovations, such as through virtual enterprise resource planning, optical character recognition for technical blueprints, and tools to guide patients through the care journey and improve outcomes. We’re beginning to see the impact of these transformations and expect leaders to outpace their peers by wider margins in the next few years.
In addition to technical change, these transformations bring together the business and cultural aspects of managing large organizations. Six core concepts underpin successful at-scale digital transformations:
- Agile @ scale. Dedicated business and technology teams come together to solve a specific problem in a matter of weeks.
- Concept sprints for a minimum viable product. Cross-functional innovation workshops focus on seizing a specific business opportunity; in them, teams use design-thinking methodologies to quickly align on tangible solutions.
- Data backbone. A scalable data backbone should establish a single authoritative source of critical information across the enterprise (for example, customer, product, pricing) and provide decision makers with business insights on demand.
- Control tower. Out-of-the-box automated infrastructure oversees the overall program and tracks the progress of more than 50 teams in real time.
- Value assurance. Teams are highly transparent and open to value audits; impact and return on investment are tracked on an ongoing basis, from inception to development and end-user adoption.
- Culture change. A focus on winning the hearts and minds of all employees ensures they embrace the new way of working and adopt digital and advanced analytics in their everyday professional lives—just as they do in their personal lives.
Digital disruption is upon us in healthcare, and medical-device industry leaders are rapidly moving from pilots and experimentation to building real capabilities at scale. We believe our four-part architecture can help you ride this wave, improve patient outcomes, and create value for all stakeholders.